Service for the Pharmaceutical Affairs Law

In addition to the business experience accumulated over 100 years, Sakata Warehouse has obtained a manufacturing license, so it is a pharmaceutical medical device related to quasi-drugs, cosmetics, and medical devices that are often requested by customers. We can prepare warehouse services and documents that comply with the law.
 Compliance with Pharmaceutical and Medical Device Act
Compliance with Pharmaceutical and Medical Device Act

Compliance with Pharmaceutical and Medical Device Act (revised Pharmaceutical Affairs Law)
The main purpose of the Pharmaceutical Affairs Law is defined as ensuring “quality,” “effectiveness,” and “safety” of pharmaceuticals, quasi-drugs, cosmetics, and medical devices. Meanwhile, the revised Pharmaceutical Affairs Law came into effect in April 2005. Immediately after the revision, there was confusion among related companies, such as different views among the health centers in each jurisdiction, but by compiling this information, we have made it possible to make proposals in accordance with the law.
Advantages of using our warehouse
- After import customs clearance of cosmetics, quasi-drugs, and medical devices, the products are brought directly to our warehouse and can be distributed in the market after a shipping judgment by the customer.
- We can perform operations that conventional logistics companies could not handle, such as replacing product packages and attaching pharmaceutical labels/enclosing efficacy notes. (This will shorten the lead time and reduce cargo handling and freight charges.)
- Our responsible engineers, who are well versed in the handling of cosmetics, quasi-drugs, and medical devices in distribution and logistics, will be stationed at the site to conduct business/quality control. (It is not necessary for the shipper (manufacturer or distributor) to have a responsible engineer on site).
- In addition, it is possible to perform work related to packaging, labeling, and storage, which previously fell under the category of manufacturing activities.
Types of manufacturing licenses obtained by SAKATA WAREHOUSE
・Cosmetics manufacturing (packaging, labeling, storage)
・Manufacture of quasi-drugs (packaging, labeling, storage)
・Medical Device Manufacturing
 Introduction of warehouses that comply with PMD Act
Introduction of warehouses that comply with PMD Act

Kansai Area
- Cosmetics manufacturing (Permit No.27CZ200768)
- Medical Device Manufacturing (Permit No.27BZ200569)
- Manufacture of quasi-drugs (Permit No.27DZ200327)
- Cosmetics manufacturing (Permit No.27CZ290030)
- Manufacture of quasi-drugs (Permit No.27DZ290022)
- Cosmetics manufacturing (Permit No.27CZ200833)
- Manufacture of quasi-drugs (Permit No.27DZ200359)
Tokyo Metropolitan Area
- Cosmetics manufacturing (Permit No.12CZ200085)
- Manufacture of quasi-drugs (Permit No.12DZ200038)
North Kanto Area
- Cosmetics manufacturing (Permit No.10CZ200126)
- Manufacture of quasi-drugs (Permit No.10DZ200080)
Chubu / Hokuriku Area
- Cosmetics manufacturing (Permit No.18CZ200011)
- Manufacture of quasi-drugs (Permit No.18DZ200002)
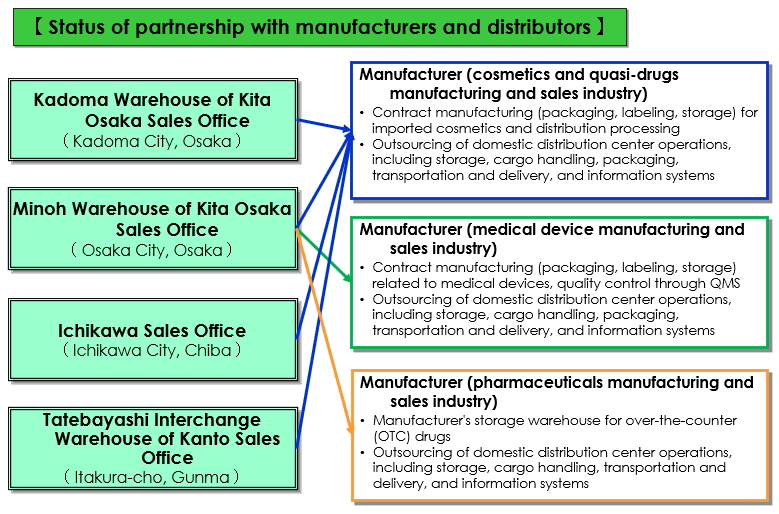
 Status of business alliances with manufacturers
Status of business alliances with manufacturers

(1) Status of business alliances with manufacturers
Note: This is the status of handling of packaging, labeling, and storage related to pharmaceutical affairs.
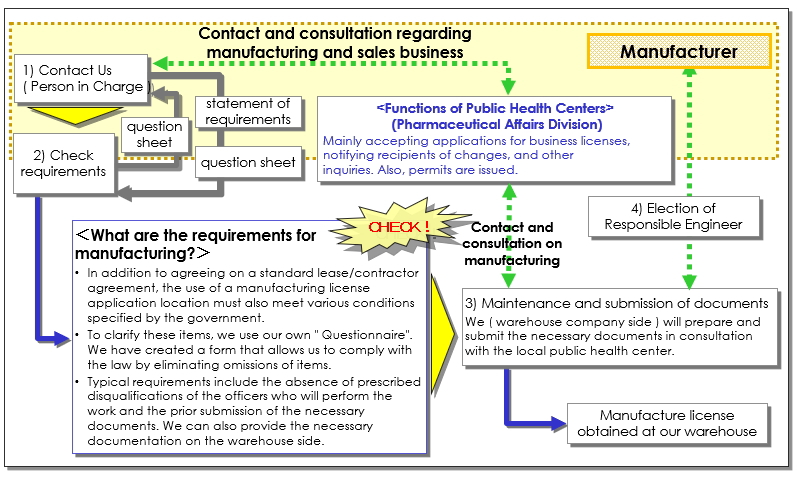
 Flow to contract
Flow to contract

(1) Flow to contract (Pharmaceutical application)
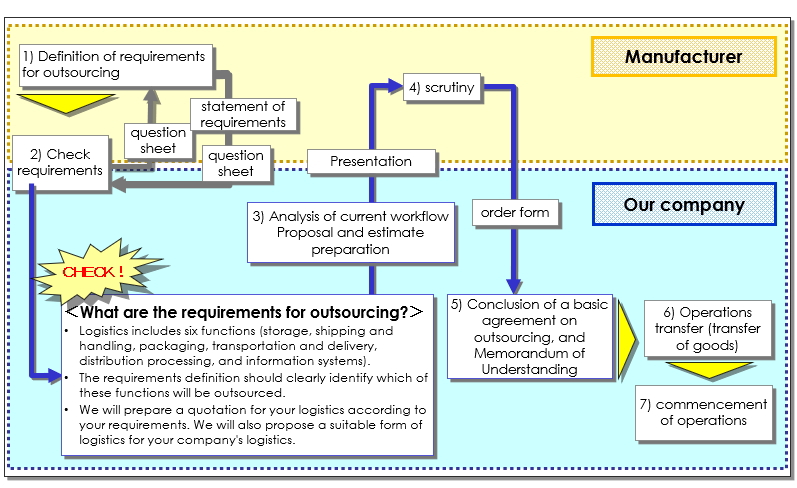
(2) Flow to contract (outsourcing)








 Compliance with Pharmaceutical and Medical Device Act
Compliance with Pharmaceutical and Medical Device Act Introduction of warehouses that comply with PMD Act
Introduction of warehouses that comply with PMD Act Status of business alliances with manufacturers
Status of business alliances with manufacturers Flow to contract
Flow to contract







